The Only Guide to Pharmaceutical Translation Services You’ll Ever Need
Let’s face it, no pharmaceutical product can enter any market without professional pharmaceutical translation services. Complex regulatory compliance, language barriers, and cultural differences are all challenges that must be navigated with care.
In this blog post, we will delve into the world of pharmaceutical and medical translation, discussing the critical role that it plays in bringing drugs to market as well as the challenges that drug developers face along the way. We’ll also explore how accurate translations are critical in clinical trials, regulatory submissions, and marketing materials, and what type of specialized translation company you should partner with.
Global Pharmaceutical Industry Market Overview
The pharmaceutical market encompasses businesses and non-profit organizations that focus on developing and selling drugs to treat illnesses and improve health. They are responsible for the research, development, production, and distribution of these medications.
The market is divided into two main segments, namely pharmaceutical drugs and biologics. Biologics comprise drugs and medicine extracted or produced from biological entities, while pharmaceutical drugs are entirely synthesized from molecular components.
As the world recovers from the Covid-19 pandemic, including the impact of social distancing, remote working, and the closure of many commercial activities, the pharmaceutical industry is finally regaining its strength. According to Grand View Research, the global drug manufacturing market was valued at USD 405.52 billion in 2020 and is expected to grow at a compound annual growth rate of 11.34% from 2021 to 2028.
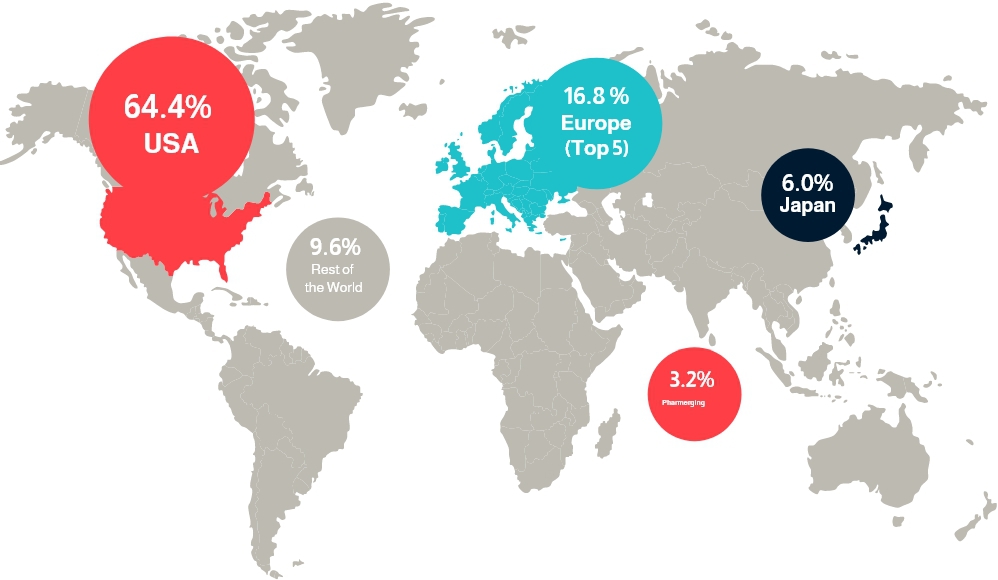
In terms of geographical breakdown, North America dominates the market with a share of 37.26% in 2020. The United States holds the dominant position in per capita prescription drug spending globally, and it is home to the largest number of pharma partnerships that are early-stage companies as well as well-established ones. Meanwhile, Asia Pacific is expected to be the fastest-growing market.
Entering the pharmaceutical market is challenging due to the high upfront costs. According to Grand View Research, the FDA approved 59 drugs in 2018, 49 in 2019, and only 15 in 2020.
Fun Fact: On average, only one to two of every 10,000 substances synthesized in laboratories will successfully pass all stages of development required to become a marketable medicine. Source: European Federation of Pharmaceutical Industries and Associations
In the pharmaceutical industry, two primary factors are driving the high demand for translation services: global expansion and marketing of drugs. If a drug is approved in one market, there’s no reason why a company shouldn’t expand into new countries and potentially leave millions on the table. This is where translation services come in to help bridge the language gap.
Furthermore, with a revenue share of more than 54% in 2020, the outsourcing sector has become dominant in the pharmaceutical industry. Manufacturers have increasingly turned to external service providers for R&D and drug manufacturing services in recent years.
Outsourcing has several advantages, including reduced investment, lower drug development, overall costs, and increased manufacturing efficiency. It also makes it easier for businesses to comply with various regulatory standards, such as the requirement for translation in regulatory compliance processes. You can read more on the demand for translation in the pharmaceutical industry below.
Why Are Translation Services Essential in the Pharmaceutical Industry?
Pharmaceutical translation services are essential for several reasons:
-
Entering New Markets
Collaboration is key in the fast-paced world of the pharmaceutical industry. Healthcare professionals, researchers, and pharmaceutical companies from all over the world collaborate to achieve a common goal: improving health and saving lives. However, with so many different languages and cultures involved, effective communication can be difficult at times. That is where professional pharmaceutical translation services come into play! It’s a necessary tool for these groups of people to communicate with one another in a clear and concise manner. For example, clinical trial translation services handle all the protocols, procedures, and trial results to ensure that the multi-national team of researchers is on the same page. As a result, researchers can work more efficiently, and the development of new medicines and treatments is thus streamlined and accelerated.
-
Regulatory Compliance
Any drug-related process is highly regulated, and pharmaceutical companies must follow various laws and regulations imposed by regulatory bodies, whether global or local. The primary goal is to ensure that these products are safe and effective. For example, if a company wants to market its product in a country that does not speak English, the pharmaceutical companies must first obtain regulatory approval in that country before they can market their products. They must submit all documentation and data pertaining to the product’s safety, efficacy, and quality in the target language. The required translations will be examined by the regulatory authorities to determine whether they meet their safety and quality control standards.
-
Customer Safety and Information
Apart from complying with regulations and ensuring product safety, pharmaceutical companies have better chances of success if they pay attention to marketing their product through the high-quality translation of all the customer-related documents. These include accurate and clear instructions on how to use medications and understand potential side effects. Companies must differentiate themselves and persuade healthcare professionals, patients, and regulators of the value of their products in the highly competitive pharmaceutical industry. One method is to be transparent and to effectively highlight the benefits of their products through a high-quality translation of all relevant documents. This transparency will help them build trust and confidence and ultimately reach a wider audience.
How Do Pharmaceutical Translation Services Shape the Processes in the Industry?
Cutting to the chase, pharmaceutical translation services are required in three main areas or processes:
- Life Scientific translation that includes clinical research and trials documentation
- Regulatory and compliance requirements to make medications eligible for any market
- Marketing translation to help sell and distribute medication
Firstly, life scientific translation involves translating a wide range of scientific documents, including clinical research and trial documentation that is part of the Research and Development (R&D) stage. Among this essential documentation are included:
- Informed Consent Forms
- Patient Information Leaflets
- Patient Literature
- Study Protocols
- Case Report Forms
- Investigator Brochures
These documents are directed at patients who will participate in the clinical trials so they need to be adapted to their cultural context.
Other audiences that will supervise and engage with these documents are multilingual and international research communities. Thus, it’s important to not only render texts word-for-word in the new target language but also pay attention to the tone of language, depending on the aim of the document.
Secondly, regulatory and compliance requirements are another critical area that requires translation services. Pharmaceutical companies must adhere to strict regulations and guidelines when developing and marketing their drugs and be able to prove it to local and international regulatory bodies in their local language.
These types of documents are very strict and require technical translation which includes expert knowledge of the bureaucratic language and the local laws. Just to mention a few, here are some documents included in regulatory submissions:
- User Manuals
- Package Inserts & Labels
- Instructions for Use
- Labelling Updates
- CE Marking Technical File (for the EU countries)
Thirdly, we come to marketing translation services in the pharmaceutical industry. The end user for medication are individuals, hospitals, clinics as well as pharmaceutical retailers.
More often than not, the translation of pharmaceutical documents with the purpose of marketing involves transcreation services.
This means most documents, especially those directed to advertise the medication in the new market will need to be created from scratch. The reason for that is that most probably the messaging you used in your source language will not fit in the new one.
Nonetheless, not every document might necessitate transcreation. Many user manuals or descriptions may require only translation but still be adapted to be culturally appropriate and accurate translations. Think of:
- Promotional materials
- Brochures
- Website Content
- Press Release
Given this level of complexity in pharmaceutical translations, it’s no surprise that most organizations rely on the services of language service providers (LSPs) who specialize in life science translation services. Partnering with the right LSP can make a significant impact on the success of your international expansion. A specialized LSP can provide not only accurate translations but also industry-specific expertise and knowledge of regulatory requirements across different countries.
However, with so many LSPs available, it can be challenging to know which one to choose. Below we give a condensed overview of what to expect.
What to Expect from High-Quality Pharmaceutical Translation Services?
For the success of your business, it is your responsibility to thoroughly research the potential of the language service provider you will partner with. But a high-quality LSP will also make your job easier for you, by being transparent about their expertise.
You should look at their record of success in translating for life science and pharmaceutical companies in specific. Working with qualified professionals who understand the requirements will drive the process faster and adhere to strict quality and confidentiality protocols as well as deadlines.
That’s why the team must include subject-matter expert teams, consisting of both project managers and translators who can handle every step of the process. Otherwise, you risk facing inconsistencies that can compromise the integrity of your project.
Language service providers should offer multilingual translation and understand the regulations governing the drug market in both the country of origin and target countries. ISO certification, especially ISO 17100 and ISO 9001, is also essential.
When choosing translators, make sure they have pharmaceutical expertise and are trained and qualified to handle your specific product. This will ensure faster and better results. And because this type of project requires precision and coherence, it’s crucial to maintain confidentiality across files and platforms
To further streamline the translation process, consider LSPs that are using intact translation management software (TMS). This will help you maintain coherence, improve cost and time efficiency as well as help you supervise and stay updated with their progress. With these factors in mind, you can rest assured that your pharmaceutical translation project will be handled with the care and attention it deserves.
Download our guide to explore 4 main
tools to increase efficiency and accuracy.
Automation has the power to transform your pharmaceutical translation workflow!
High-Quality Pharmaceutical Translation Services Checklist

Conclusion
The significance of professional pharmaceutical translation services cannot be overstated. Accuracy and effectiveness are critical for pharmaceutical product development, marketing, and regulation, and Laoret understands this better than anyone. With our extensive experience in the pharmaceutical industry, we provide a variety of services tailored to your specific requirements, such as life science, regulatory, and marketing translation. At Laoret, we value precision and adherence to industry standards, and we are committed to providing our clients with exceptional service. As an ISO-certified translation company, Laoret guarantees the highest quality translations that meet global standards. In addition, our team employs translation management software to optimize the translation process, resulting in improved cost and time efficiency. Request a quote to learn more.
References
- Statista: The world’s most spoken languages
- World Data: The world’s largest economies
- Internet world users by language
- The 10 Largest E-Commerce Markets in the World by Country
- English levels in China
- The most used languages on the internet
- China: Language simplification to increase literacy?
- The main differences between Mandarin and Cantonese
- The Spanish language in the world
- Internet world users by language
- The U.S. Has the Second-Largest Population of Spanish Speakers’ How To Equip Your Brand To Serve Them
- Parker pens make you pregnant, and other due diligence fails!
- Arabic Speaking Countries
- Arab economies to post 5.4 percent growth rate this year on higher oil prices
- More Arab countries are seeking to orient their economies towards knowledge
- Individuals using the Internet (% of population) – Arab World
- French speaking countries
- English Loses Currency as Europe’s Lingua Franca After Brexit Vote
- The rise of Africa’s digital economy
- Mechanical Engineering Industry in Germany: Our Industry Report
- Internet user penetration in Germany from 2018 to 2027






