Key Tactics to Navigate IFU Technical Language Translation
Getting medical devices into a foreign-speaking market comes with the responsibility of submitting accurate IFU technical language translations for seamless premarket approval and device distribution.
Besides the strict regulatory framework, the complexities of the technical language involved be daunting and challenging to navigate.
IFU translation must check all the boxes regarding linguistic accuracy and technical precision, which calls for top-notch language proficiency and expertise in the medical field.
Today, we will tackle everything about IFU translation services from its significant role to key factors and challenges. Also, we will provide tips and tricks to help simplify the technical language translation process.
So, let’s dive in!
Why Is IFU Translation So Important?
Before delving into the challenges of IFU technical language translation, let us address the fundamental role it plays in driving the success of medical device companies.
Market Expansion and IFU Legislation
As medical device companies are expanding their reach and growing their market share, they have two missions at hand:
- Introducing new products into already established markets.
- Cross-border distribution targeting new foreign-speaking markets.
To get medical devices on the market, relevant regulatory bodies must approve the Instructions For Use (IFU), device labeling, and packaging before distribution.
For instance, the FDA (Food and Drug Administration) is a federal agency of the Department of Health and Human Services in charge of regulating the sale of medical devices and pharmaceutical products in the US. As such, the FDA must approve the IFU and labeling to ensure medical device conformity and user safety.
The FDA has language requirements for medical device labeling that go as follows:
“All labeling shall be in English with the exception of products distributed solely within Puerto Rico or a U.S. territory where the predominant language is other than English. In these instances, the predominant language may be substituted for English. If any representation on the device label or labeling appears in a foreign language, then all required labeling shall also appear in that foreign language.”
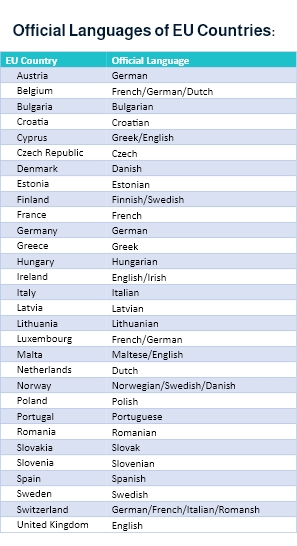
Moreover, when venturing into the European market, medical device companies must comply with the EU MDR ( European Union Medical Device Regulation) requirements, where translations must be in the official language determined by the EU member state.
As per the EU MDR,
“Manufacturers shall ensure that the device is accompanied by the information set out in Section 23 of Annex I in an official Union language(s) determined by the Member State in which the device is made available to the user or patient. The particulars on the label shall be indelible, easily legible, and clearly comprehensible to the intended user or patient.”
Additionally, “Manufacturers shall, upon request by a competent authority, provide it with all the information and documentation necessary to demonstrate the conformity of the device, in an official Union language determined by the Member State concerned.”
While IFU translation might take time and effort, it evidently plays a significant role in streamlining the process of cross-border distribution.
Effective Use and Patient Safety
Facilitating the effective use of medical products and reducing health risks is a top priority in the medical device landscape. For this reason, it’s the medical device manufacturer’s responsibility to provide clear IFU translation in the end user’s native language across different markets.
But with such responsibility comes great benefits.
For one, multilingual IFU translation provides an effective user-centered experience. When the IFU is in the user’s native language, they can clearly read and understand the instructions with no room for misinterpretation or misunderstanding. Accordingly, they can use the product easily, safely, and confidently, being fully aware of safety precautions, risks, and proper usage.
What’s more, when medical device companies translate IFUs as a risk management strategy, they minimize the risk of financial and legal liabilities that can result from misinterpretations and errors in product use due to language barriers. This commitment to user safety and satisfaction enhances the manufacturer’s reputation and brand perception among consumers in these new markets.
Fun Fact: IFU translation is only one part of the medical device compliance process.
If you want to learn more about the intricate process of medical device documentation, check out our medical device lifecycle guide.
This guide outlines the medical device document translation process – from research to distribution.
IFU Technical Language Translation: Key Factors and Challenges
The technical nature of medical device translation, and IFU in particular, poses a challenging task for medical device manufacturers and translators.
- The complexity of IFU content mainly lies in its critical technical context. IFUs can describe intricate, step-by-step processes that users must follow. Think of it like explaining how to assemble a complex piece of furniture.
When translated into a different language, each step should remain clear and comprehensible, while preserving the sequence. A single misplaced or misunderstood step can lead to errors or even safety hazards.
- Furthermore, IFU uses complex terminology that involves specialized abbreviations and acronyms. The thing about medical and technical jargon is that it can convey different meanings depending on the context.
This explains why in-depth knowledge of medical and technical contexts is essential to correctly comprehend the text and deliver terminologically accurate translations.
- Another challenging aspect to keep in mind is the cultural preferences and local standards. The use of measurement units and numbers is important to explain dosage information and other instructions.
This demands attention to cultural sensitivities. Adapting these elements to align with local preferences is a must to ascertain that target users can comprehend information correctly.
How to Navigate the Complexities of IFU Technical Language Translation
As a rule of thumb, IFU translation must be technically precise, terminologically accurate, and user-friendly.
Here are 5 tactics to help you achieve that and deliver simplified and accessible translations!
1. Target Audience
A comprehensive understanding of the target demographic and national regulations provides practical guidance on how to approach IFU technical language translation.
Before jumping into the translation process, begin by thoroughly understanding who the end users of the translated IFU are and accordingly tailor the language and terminology to match the users’ comprehension level.
Medical device companies cater to a wide range of users, from medical professionals to laypersons. For instance, healthcare professionals may require a different level of technical detail compared to general consumers. For them, you can use more specialized terms, but for general consumers, opt for simpler, everyday language.
Additionally, consider their education level, language proficiency, cultural background, and familiarity with the product.
A Survey of US Adults found that 36% of respondents had basic or below-basic health literacy. Limited health literacy lowers patients’ ability to understand and correctly follow treatment instructions, even when communicated in their native language!
If the audience includes speakers of multiple languages, ensure that translations are adapted to the literacy levels and cultural sensitivities of each group.
Pro Tip!
The FDA suggests using a language proficiency that doesn’t exceed the national average reading level for patient-use instructions.
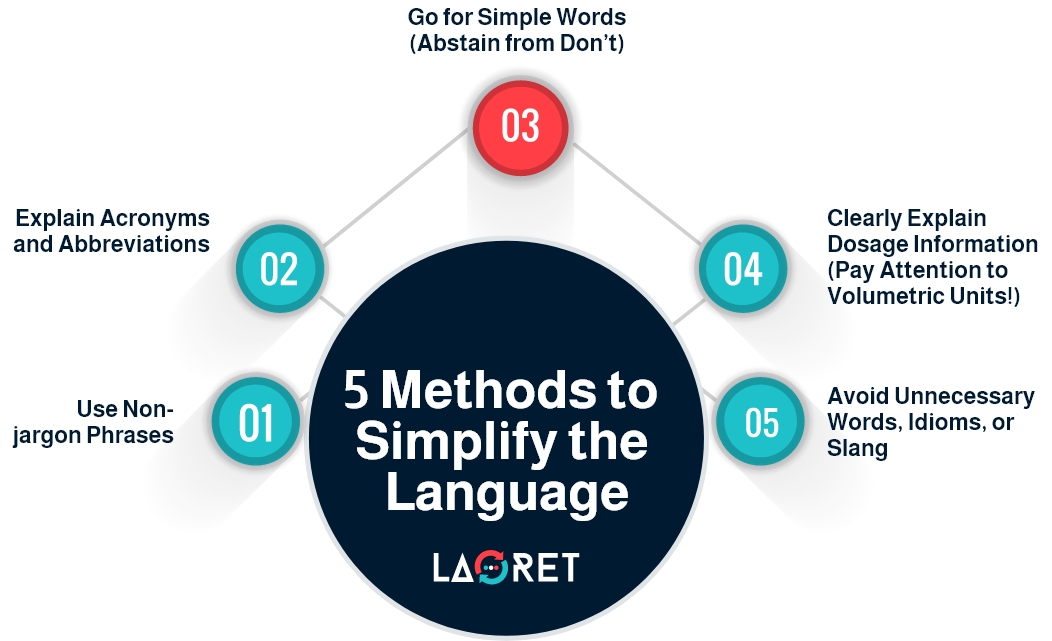
2. Simplify Technical Language
A study on patients’ interpretation of common medical phrases found that using “non-jargon” phrases in a medical setting remarkably improved patients’ understanding and reduced confusion.
Use plain and straightforward language to explain complex concepts and determine which technical terms are essential for users to know and which can be simplified or omitted. Technical terms that directly impact the safe and effective use of the product should be retained and explained. For this reason, simplifying the technical jargon in patient-use IFU is the first step to ensure correct interpretation and reduce medical errors.
Moreover, user manuals typically use specialized acronyms and abbreviations that the average user is not familiar with. That’s why, translators should break down technical acronyms using consistent terms and phrases.
Also, for dosage information, translators must employ user-friendly terms and break down the preparation instructions step-by-step. More importantly, it’s necessary to adhere to local standards when adapting volumetric units and numerals to fit the target locale.
Apart from the technical terminology, the overall language should be simple and accessible. For instance, using “Don’t” or “Avoid” rather than “Abstain from”. It is best to keep the text straight to the point, eliminating any unnecessary words or idioms.
That being said, it’s important to highlight that translators must maintain technical precision in the process of simplifying the technical language. You can’t use slang or unclear idiomatic expressions.
Most importantly, include a glossary or definitions section in the IFU for technical terms that must be retained but may not be commonly understood by users. This allows users to refer to definitions as needed for clarity.
3. Active Voice
Active voice is where the subject performs the action, while passive voice is where the action is done to the subject. Passive voice can sometimes make instructions sound less clear and more complicated, so it’s best to favor active voice for user-friendly IFUs.
IFU contains action-oriented detailed instructions. To make them easily comprehensible, translators should use clear-cut command language and active voice throughout the document. In fact, the FDA recommends starting sentences with an action verb whenever feasible.
For example, instead of saying “You are advised to avoid strenuous exercise”, it is better to say, “Avoid strenuous exercise”.
Using the active voice in translations makes instructions more direct and easier to follow. It leaves no room for ambiguity regarding who should take action.
4. Clear and Concise Explanations
We all know how overwhelming reading large volumes of text can be, and IFUs are no exception. Medical device IFU includes detailed information about the intended use, administration, side effects, storage, disposal, and cautionary warnings.
On one hand, translators should clarify all relevant information eliminating any ambiguities that might cause confusion or incorrect use of medical devices.
On the other hand, it’s paramount to produce translations that are easy to read for the average user. After all, you don’t want to deter patients from reading it, right?
So, it is best to keep the translations clear and concise. But how can you achieve that?
- First, don’t assume that patients are familiar with the device and its instructions. It is best to use logically convincing language to explain the “WHY” and “HOW”. Leave no room for ambiguity or speculation.
- Organize information hierarchically, with the most critical instructions and warnings prominently displayed. Use headings, bullet points, and numbering to structure the content effectively. This hierarchy helps users locate and prioritize information.
- Maintain consistency. Use consistent terms, titles, and layout formatting throughout the document to avoid confusing the reader.
- Keep it short! Break down long paragraphs into shorter sentences and make use of bullet points and lists to help readers digest important information easily and quickly.
- Incorporate visual illustrations, symbols, and icons to better explain complicated steps.
5. Visual Aids, Symbols, and Readability
Using visual aids and culturally relevant symbols as well as preserving original spacing can further improve the technical language translation and enhance readability. However, it’s critical to consider different cultural nuances and linguistic complexities that influence the file format and readability.
Now, here are two things to consider in the process:
- Adapt visual elements, symbols, or icons and make sure they’re culturally relevant and aligned with local conventions.
- Perform Desktop Publishing (DTP) to preserve the original layout and fix any disarrangement resulting from text expansion and contraction.
The translated text could significantly alter the original format if it occupies more or less space than the source text. That’s because the number of words or characters used to convey the same meaning differs from one language to another.
As such, DTP specialists and designers must work on the post-translation document and perform formatting checks before printing or publishing. This is important to ascertain that the file is perfectly aligned and easily comprehensible for end users.
Overwhelmed? We’ve prepared a quick guide for you!
Our compact “Key Tactics for User-Friendly IFU Translation” guide provides key tips and tricks to help you deliver IFU translations that are compliant and user-friendly.
Streamline Your Market Expansion Plans with Laoret’s IFU Translation Services!
Laoret’s certified life science translation services offer international compliance and subject-matter expertise in over 120 languages.
We strictly apply rigorous Quality Assurance procedures in accordance with ISO 17100 and ISO 9001. With cutting-edge technology and a multidisciplinary team of native linguists and medical reviewers, Laoret is poised to handle your IFU translation project with the utmost accuracy and efficiency.
Request a quote now and get closer to global success!






